Pipeline
Our Pipeline is comprised of Biologics, including the proprietary BEAT® MultispecificsTM platform, and one Cbl-b inhibitor small molecule targeting the spectrum of hematological cancers and solid tumors. If you are interested in exploring a partnership, Contact us.
Oncology
- Overview
- ISB 2001
- GRC 65327
Diversity of Immune Cell Engagement and Indications across Hematologic and Solid Tumors
| ASSETS | DESCRIPTION | INDICATION |
|---|---|---|
| CLINICAL ASSETS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
ISB 2001
BCMA x CD38 x CD3 TREAT ™
Trispecific T-Cell Engager
PHASE 1
ORPHAN DRUG
GRC 65327
Cbl-b Inhibitor Small Molecule
Solid Tumors
PRECLINICAL
| CANDIDATES |
|---|
ISB 2301
IMMUNITE™
NK-Cell Engager
Solid Tumors
DISCOVERY
PRODUCTS
COMPOUND
CLINICAL ASSETS
ISB 2001
TARGET
BCMA x CD38 x CD3 TREAT ™
trispecific T-Cell Engager
INDICATION
Multiple Myeloma
PHASE
STATUS :
PHASE 1 ORPHAN DRUG
PRODUCTS
COMPOUND
CLINICAL ASSETS
GRC 65327
TARGET
Cbl-b Inhibitor Small Molecule
INDICATION
Solid Tumors
PHASE
STATUS :
PRE-CLINICAL
PRODUCTS
COMPOUND
CANDIDATES
ISB 2301
TARGET
IMMUNITE™
NK-Cell Engager
INDICATION
Solid Tumors
PHASE
STATUS :
DISCOVERY
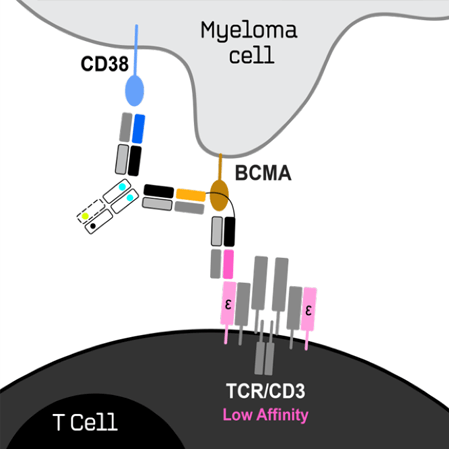
ISB 2001 is first TREATTM Trispecific Antibody for
Relapsed/Refractory Multiple Myeloma
- BCMA and CD38 are expressed on the surface of multiple myeloma cells and are clinically validated targets.
- ISB 2001 combines three proprietary Fab arms binding to CD3 on T-cells, and to BCMA and CD38 on myeloma cells.
- In vitro studies showed increased killing potency of tumor cells compared to all tested antibodies, including currently approved and investigational multiple myeloma therapies.
- In vivo studies in multiple myeloma models also show superior potency relative to antibodies for the treatment of multiple myeloma.
- ISB 2001 redirects CD3+ T lymphocytes to kill tumor cells expressing from low
to high levels of both BCMA and CD38. - With two different tumor-associated antigens, ISB 2001 is expected to be more resistant to antigen escape associated with treatment of MM patients
. - CLINICAL DATA
Clinical Data from part I (dose escalation) of the TRIgnite-1 study were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in June 2025 (Link). The results demonstrated high overall response rates, with deep and durable responses in heavily pretreated patients with Relapsed/Refractory Multiple Myeloma – regardless of prior exposure to T-cell engagers (TCE) or CART-cell therapies. The safety profile was favorable, with most side effects being low-grade and manageable.
GRC 65327 A Novel Small Molecule
Selective Oral Cbl-b Inhibitor Immunotherapeutics
- Selective, small molecule, orally available, Cbl-b inhibitor, phase I-ready for solid tumor indications.
- Demonstrated nM Cbl-b activity, >20-fold selectivity, potentiation of IL-2 and IFN-γ and T cells proliferation.
- Significant tumor growth inhibition as a monotherapy and in combination with anti-PD1, while also inducing durable complete responses associated with memory immune responses.
- In a 1-month GLP monkey toxicology study, an increased T cells activation and infiltration was observed in mesenteric lymph nodes (a tissue immune response) at very low exposures of AUC ~1500 ng.h/mL and above.
- IND submission to DCGI completed in October 2024

For collaborations, please contact us here.
Diversity of Immune Cell Engagement and Indications across Hematologic and Solid Tumors
| ASSETS | DESCRIPTION | INDICATION |
|---|---|---|
| CLINICAL ASSETS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
ISB 2001
BCMA x CD38 x CD3 TREAT ™
Trispecific T-Cell Engager
PHASE 1
ORPHAN DRUG
GRC 65327
Cbl-b Inhibitor Small Molecule
Solid Tumors
PRECLINICAL
| CANDIDATES |
|---|
ISB 2301
IMMUNITE™
NK-Cell Engager
Solid Tumors
DISCOVERY
PRODUCTS
COMPOUND
CLINICAL ASSETS
ISB 2001
TARGET
BCMA x CD38 x CD3 TREAT ™
trispecific T-Cell Engager
INDICATION
Multiple Myeloma
PHASE
STATUS :
PHASE 1 ORPHAN DRUG
PRODUCTS
COMPOUND
CLINICAL ASSETS
GRC 65327
TARGET
Cbl-b Inhibitor Small Molecule
INDICATION
Solid Tumors
PHASE
STATUS :
PRE-CLINICAL
PRODUCTS
COMPOUND
CANDIDATES
ISB 2301
TARGET
IMMUNITE™
NK-Cell Engager
INDICATION
Solid Tumors
PHASE
STATUS :
DISCOVERY
ISB 2001 is first TREATTM Trispecific Antibody for
Relapsed/Refractory Multiple Myeloma
- BCMA and CD38 are expressed on the surface of multiple myeloma cells and are clinically validated targets.
- ISB 2001 combines three proprietary Fab arms binding to CD3 on T-cells, and to BCMA and CD38 on myeloma cells.
- In vitro studies showed increased killing potency of tumor cells compared to all tested antibodies, including currently approved and investigational multiple myeloma therapies.
- In vivo studies in multiple myeloma models also show superior potency relative to antibodies for the treatment of multiple myeloma.
- ISB 2001 redirects CD3+ T lymphocytes to kill tumor cells expressing from low
to high levels of both BCMA and CD38. - With two different tumor-associated antigens, ISB 2001 is expected to be more resistant to antigen escape associated with treatment of MM patients
. - CLINICAL DATA
Clinical Data from part I (dose escalation) of the TRIgnite-1 study were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in June 2025 (Link). The results demonstrated high overall response rates, with deep and durable responses in heavily pretreated patients with Relapsed/Refractory Multiple Myeloma – regardless of prior exposure to T-cell engagers (TCE) or CART-cell therapies. The safety profile was favorable, with most side effects being low-grade and manageable.

Inflammation and Autoimmune Disease
The autoimmune disease assets have been out-licensed to enable greater focus on oncology. Explore the pipeline chart below to learn more and Contact Us for additional information.
Autoimmune Disease
PRODUCTS
Telazorlimab (and ISB 830-X8)
DESCRIPTION
OX40 antagonist
Monoclonal Antibody
Atopic Dermatitis*
| PRODUCTS | DESCRIPTION | |
|---|---|---|
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
Licensed to
$320 million for upfront payment, development, regulatory and sales milestone payments,
plus tiered royalties on global sales
Licensed to
€20.8 million for upfront payment. Plus development, regulatory and sales milestone payments,
and tiered royalties on global sales



