Partnerships
Let’s co-create to deliver cutting-edge solutions
We believe that combining forces will help us deliver innovative solutions at an accelerated pace.
IGI invites collaboration with partners who share the same belief and can complement our capabilities to propel forward innovations in cancer therapy. We seek to collaborate through, both, out-licensing and in-licensing opportunities.
Partnerships for Oncology Pipeline
IGI is committed to advancing its clinical-stage oncology portfolio. We would like to join hands with partners who can help us fast-track our mission of bringing innovative therapeutics to patients with hematologic cancers and solid tumors.
- Overview
- GRC 65327
- ISB 2301
Diversity of Immune Cell Engagement and Indications across Hematologic and Solid Tumors
| ASSETS | DESCRIPTION | INDICATION |
|---|---|---|
| PRODUCTS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
PRODUCTS
COMPOUND
GRC 65327
TARGET
Cbl-b inhibitor small molecule
INDICATION
Solid Tumors
PHASE
PRODUCTS
COMPOUND
ISB 2301
TARGET
IMMUNITE™ multimodal immunotherapy
INDICATION
Solid Tumors
PHASE
STATUS : PRECLINICAL
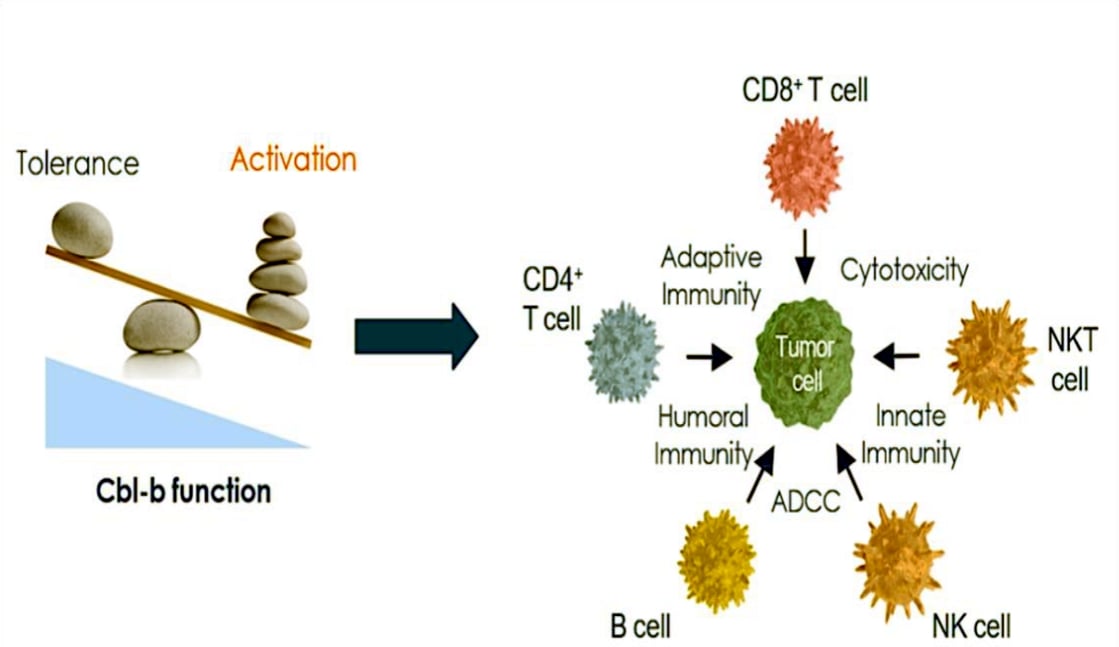
GRC 65327 A Novel Small Molecule
Selective Oral Cbl-b Inhibitor Immunotherapeutics
- Selective, small molecule, orally available, Cbl-b inhibitor, phase I-ready for solid tumor indications.
- Demonstrated nM Cbl-b activity, >20-fold selectivity, potentiation of IL-2 and IFN-γ and T cells proliferation.
- Significant tumor growth inhibition as a monotherapy and in combination with anti-PD1, while also inducing durable complete responses associated with memory immune responses.
- In a 1-month GLP monkey toxicology study, an increased T cells activation and infiltration was observed in mesenteric lymph nodes (a tissue immune response) at very low exposures of AUC ~1500 ng.h/mL and above.
- IND submission to DCGI completed in October 2024
ISB 2301
- ISB 2301 is a first-in-class NK cell-engager developed for solid tumors and the first program from IGI’s IMMUNITE™ platform
- Program is in the IND-enabling stage
- IP filing on-going
- MoA to be released during CY2026
For collaborations, please contact us here.
Diversity of Immune Cell Engagement and Indications across Hematologic and Solid Tumors
| ASSETS | DESCRIPTION | INDICATION |
|---|---|---|
| PRODUCTS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
PRODUCTS
COMPOUND
GRC 65327
TARGET
Cbl-b inhibitor small molecule
INDICATION
Solid Tumors
PHASE
PRODUCTS
COMPOUND
ISB 2301
TARGET
IMMUNITE™ multimodal immunotherapy
INDICATION
Solid Tumors
PHASE
STATUS : PRECLINICAL
GRC 65327 A Novel Small Molecule
Selective Oral Cbl-b Inhibitor Immunotherapeutics
- Selective, small molecule, orally available, Cbl-b inhibitor, phase I-ready for solid tumor indications.
- Demonstrated nM Cbl-b activity, >20-fold selectivity, potentiation of IL-2 and IFN-γ and T cells proliferation.
- Significant tumor growth inhibition as a monotherapy and in combination with anti-PD1, while also inducing durable complete responses associated with memory immune responses.
- In a 1-month GLP monkey toxicology study, an increased T cells activation and infiltration was observed in mesenteric lymph nodes (a tissue immune response) at very low exposures of AUC ~1500 ng.h/mL and above.
- IND submission to DCGI completed in October 2024

BEAT® Platform Partnerships
IGI is constantly striving towards advancement of the next wave of discovery-stage assets.
Our BEAT® platform enables deep exploration of the bi/multispecific design space to optimize drug candidates and unlock new biology including T-cell, NK-cell, and macrophage engagers.
The BEAT® Features include:
- Heavy chain pairing technology
- Heavy/light chain pairing technology using a common light chain
- Proprietary tools for selection of high-quality antibodies (phage and mammalian display)
- Effective target affinity and avidity maturation
- Efficient purification aided by differential binding to Protein A, 95% yield in a single purification step
- Flexible platform enables exploration of the full design space
- Fc function activity can be modulated (T-cell: silent; non T-cell: active or enhanced)
- Comprehensive screening capabilities enabling fast identification of leads with best functional activity and drug-like properties
Oncology
In a major strategic milestone (press release), IGI entered into a global licensing agreement with AbbVie for its lead asset, ISB 2001, a first-in-class trispecific T-cell engager currently in Phase 1 clinical development for relapsed/refractory multiple myeloma, with potential for treating certain autoimmune diseases. Under the terms of the agreement, AbbVie will receive exclusive rights to develop, manufacture, and commercialize ISB 2001 across North America, Europe, Japan, and Greater China. IGI has received an upfront payment of $700 million and is eligible to receive up to $1.225 billion in development, regulatory, and commercial milestone payments, along with tiered, double-digit royalties on net sales.
PRODUCTS
ASSETS
ISB 2001
DESCRIPTION
CD38 x BCMA x CD3
T-Cell Engager Trispecific
INDICATION
Multiple Myeloma
PHASE
STATUS
PHASE 1b
| ASSETS | DESCRIPTION | INDICATION |
|---|---|---|
| PRODUCTS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
ISB 2001
(ABBV-2001)
Autoimmune Disease Partnerships
IGI has out-licensed two assets that were developed to treat a range of autoimmune diseases with high unmet need.
Autoimmune Disease
PRODUCTS
Telazorlimab
DESCRIPTION
OX40 antagonist
Monoclonal Antibody
Atopic Dermatitis*
Asset/ MOA
ISB 880 (LAD191)
STATUS
IL-1RAP antagonist
Monoclonal Antibody
Hidradenitis Suppurativa
| PRODUCTS | DESCRIPTION | |
|---|---|---|
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
Licensed to
$320 million for upfront payment, development, regulatory and sales milestone payments,
plus tiered royalties on global sales
Licensed to
€20.8 million for upfront payment. Plus development, regulatory and sales milestone payments,
and tiered royalties on global sales
Ready to join hands?
In-licensing
We are also open to explore any in-licensing opportunities.
Our scale and speed of clinical trials enable us to work with partners who are seeking solutions for an array of medical challenges. We are equipped to collaborate with them at various stages of product development, across the disease spectrum. Furthermore, with easy access to study participants in India and across the globe, we bring a unique advantage to our partners who endeavor to expedite the trials.
We seek partners who have
Differentiated assets in
hematology-oncology and
solid tumors

Innovative technologies
and clinical-stage assets

Aim to access the large
and growing market
in India



